class: right, bottom, white, title-slide .title[ # <strong>Wildlife Diseases</strong> ] .subtitle[ ## EFB 390: Wildlife Ecology and Management ] .author[ ### Dr. Elie Gurarie ] .date[ ### November 7, 2024 ] --- <!-- https://bookdown.org/yihui/rmarkdown/xaringan-format.html --> .pull-left-60[ ## First, some slides on Body Condition **Fecundity** and **Survival** both depend very much on **body condition**. **Body condition** is measured in many ways, for example:. > .small[ We analysed two metrics of body condition: i) a direct subcutaneous **fat score (categorical, 1-5)**, assessed by palpating the belly-loin, with the badger laterally recumbent, and ii) a **body condition index (BCI)** estimated using a ratio-based approach for each capture: log (body mass)/log (body length). ] European badger (*Meles meles*): .pull-right-50[] ] .pull-right-40[ <br><br> 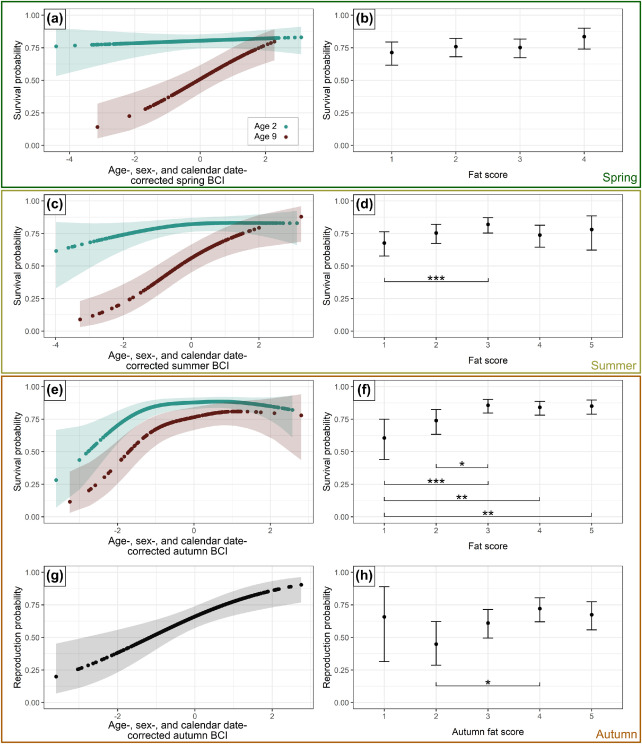 .center[[(Ross et al. 2021)](https://www.sciencedirect.com/science/article/pii/S2666900521000228)] ] --- ## Remote assessments of body condition .pull-left-30[ Field measurements made using categorical assessments. ] .pull-right-70[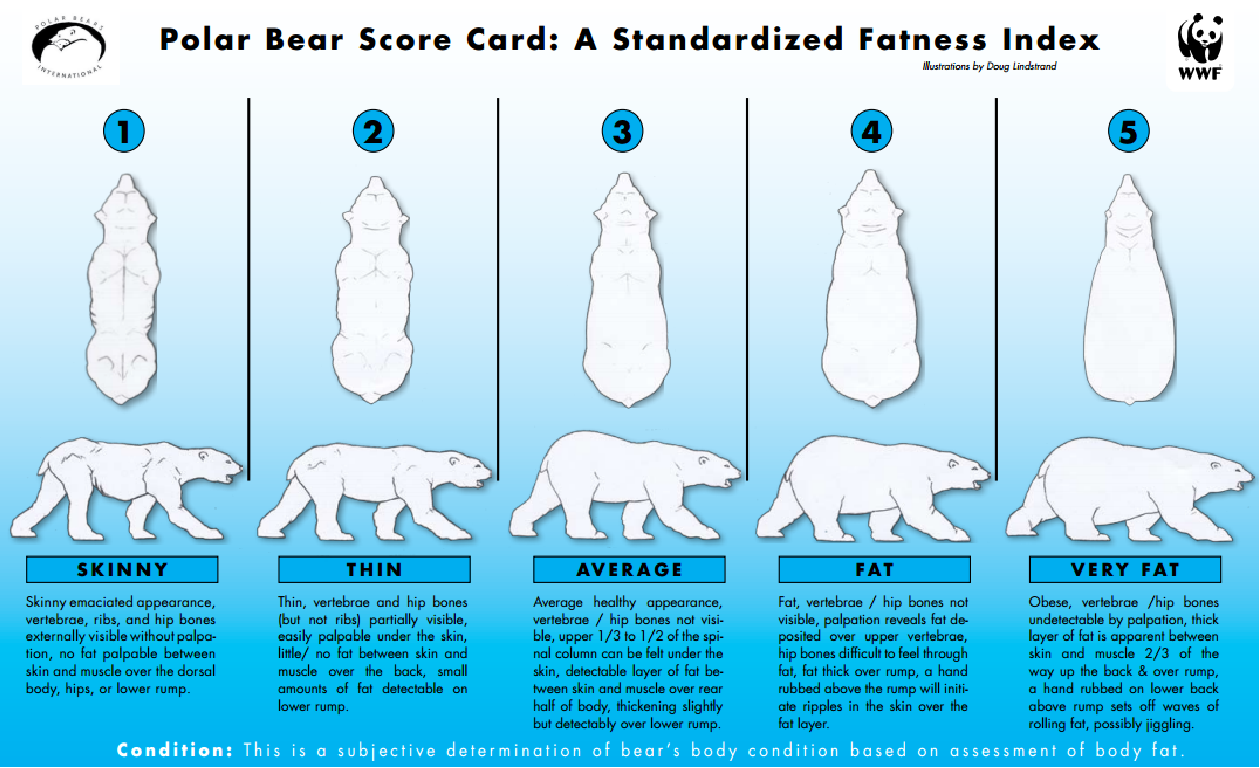] --- .pull-left-30[ ## Other measurements #### Note invasive measures Kidney fat - healthy mammals Bone marrow fat - informative for non-healthy mammals ] .pull-right-70[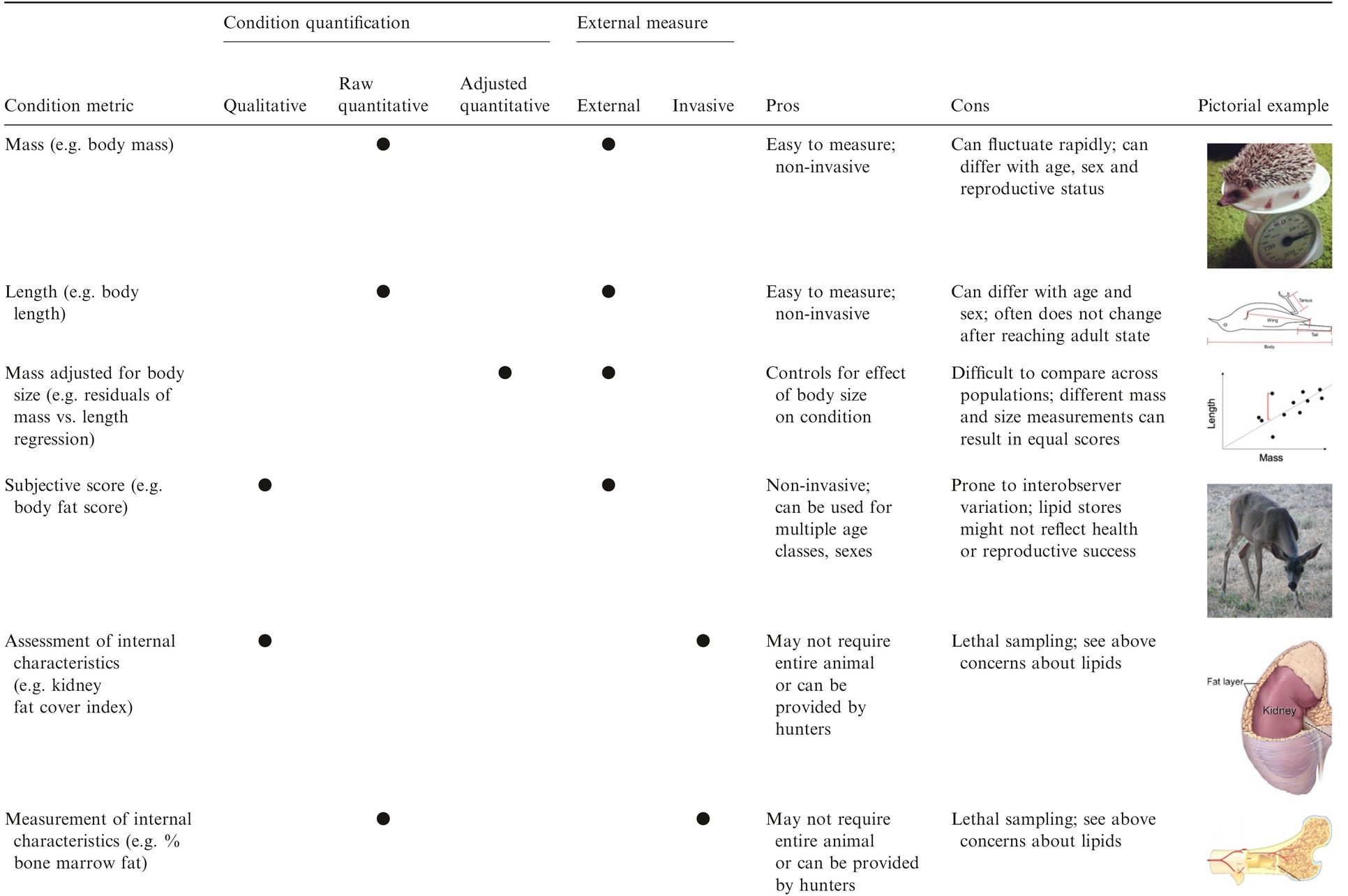 .center[([Sanchez et al. 2018](https://onlinelibrary.wiley.com/doi/epdf/10.1111/ele.13160))] ] --- # Considerations of measuring body condition .pull-left-40[ Short- and long-term effects of a stressor all need to be considered, and are measured differently (and have different consequences).  .center[***Any idea what this is!?***] ] .pull-right-60[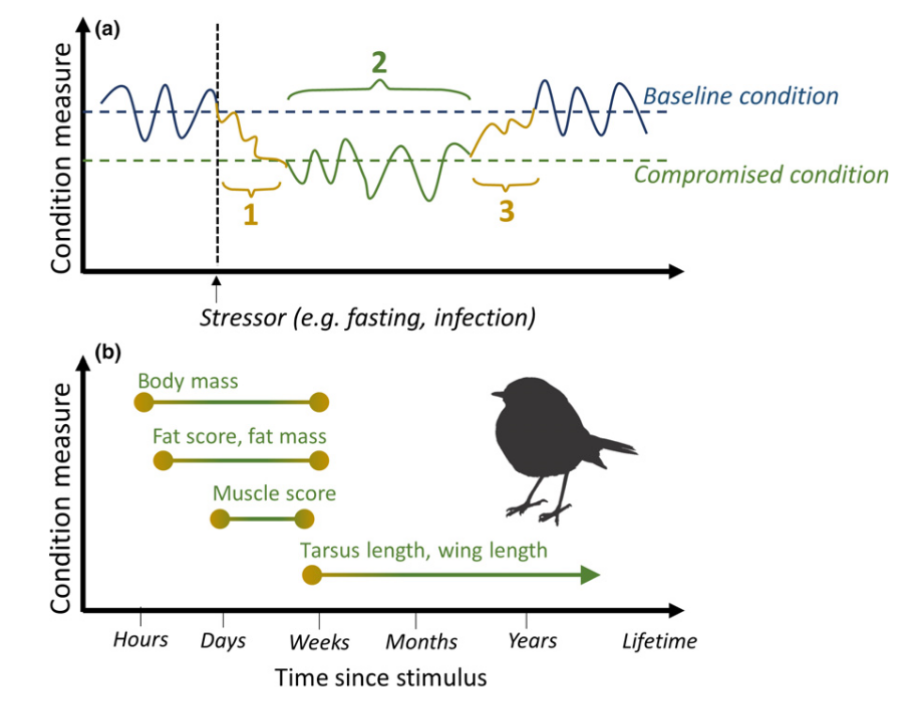] --- .pull-left[ ## Influences on body condition - **Food availability** - including (often) high seasonal variation - These relationships make **Body Condition** a good proxy for **Habitat Quality** - **Disease and parasite loads** - **Stress** - from human, predator impacts, or social interactions ] .pull-right[ ## Consequences of (poor) body condition - Lower **survival** & lower **fecundity** - Varies by age - Often - physiological energetic trade-offs between **survival** and **reproduction** (less gonadal growth, later maturation, skipped estrus) - Population level impacts - mechanisms of **Density Dependence** ] --- background-image: url("images/Chytridiomycosis.jpg") background-size: cover class:inverse #Back to Wildlife Disease --- .pull-left[ ## Why do we care? .large[ They affect humans - **Wildlife** <-> **Human** transmission They affect animals - Population = Birth + Immigration - Emigration - **DEATH** **Pathogens** are also part of the ecosystem! ]] -- .pull-right[ ## Disease Ecology is super interesting! .large[ .pull-left[ - Ecology - Behavior - Medicine - Evolution - Genetics - Immunology - Epidemiology - Modeling - Trophic Interactions ] .pull-right[ - Hosts - Intermediate hosts - Pathogens - Predator-mediation - Biotic/abiotic environments - Climate Change ]]] --- <iframe src="https://www.youtube.com/embed/o5ECFLG2EwI?controls=0" width="900" height="600" frameborder="0" allowfullscreen> </iframe> <!-- https://www.youtube.com/watch?v=59STc4gg4wI&ab_channel=WyomingGameandFishDepartment--> --- class: large ## Some Definitions ### **Pathogen:** infectious biological agent that causes disease or illness to its host #### .large[Bacteria | Viruses | Fungi | Other] ### **Vector:** a host that can transmit but is not itself affected by a pathogen ### **Zoonotic Disease:** a human disease of wildlife origin ### **Natural Reservoir:** population of organisms or specific environment in which an infectious pathogen lives and reproduces ### **Infectivity:** ability of a pathogen to establish an infection. ### **Virulence / Pathogenicity:** (severe or fatal cases) / (total cases) --- background-image: url("images/rabid_dog.webp") background-size: cover # .yellow[**Zoonotic diseases**] ### .yellow[(because they affect humans)] -- .pull-left[] --- ## Zoonotic diseases .pull-left-40[ **Emerging infectious diseases per decade**  ] .pull-right-60[ 60% of EID are caused by zoonotic pathogens. 72% of zoonotic EID origins in **wildlife** (vs. domesticated) origin.  **Note prominence of NE. US!** ([Jones et al. 2008](https://www.nature.com/articles/nature06536)) ] --- .pull-left-40[ ## Example: **Rabies**  .small[http://europepmc.org/article/PMC/6899062] ] .pull-right[ <br> **Rabies** is an *encephalitis* (brain inflammation) for *most* mammals. Spread by *Lyssavirus* (family of viruses) spread through saliva. The disease (cleverly!) modifies behavior to make spread via saliva more likely: - aggressiveness leading to biting. - excrutiating pain when swallowing / hydrophobia, Largely **asymptomatic** bat *reservoir*, leaks into spillover infections to, esp., domestic dogs & humans. **Effective vaccines exist!!** (in fact, developed by Louis Pasteur in 1885) ] --- .center[## Rabies vectors] .pull-left[ In the US (**where rabies is very very rare**) - almost all carriers are wild animals. Variants are (usually) species-specific. Wild animal vectors depend on local ecology.  .small[ [www.cdc.gov](https://www.cdc.gov/rabies/location/usa/surveillance/wild_animals.html) ] ] .pull-right-50[  ] --- .pull-left[ ## How to manage **Rabies**? - Dog management (stray control / muzzling / vaccination) - Vigilance - Removal of infected animals - Vaccination! (trap / release or oral)  ] -- .pull-right[  ### Including the wild ones ] --- .pull-left-60[ ## Example: **Lyme Disease** 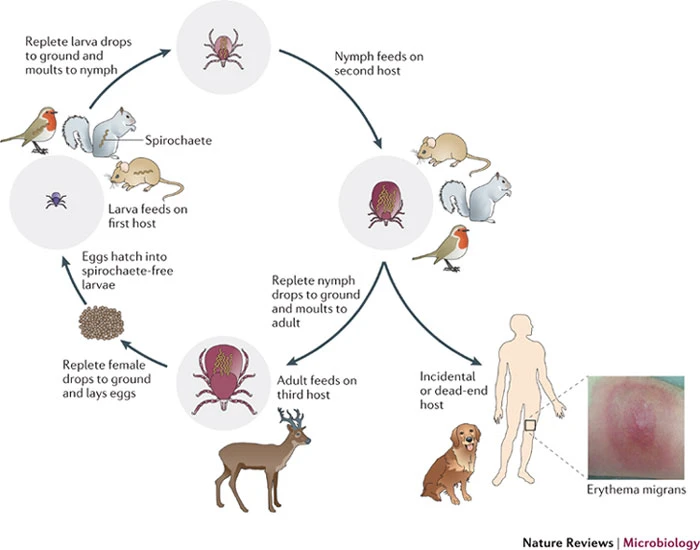 .small[Nature.com] ] .pull-right-40[ ### Most prevalent vector-borne disease in N. America **Pathogen:** *Borrelia burgedorferi* (bacteria) Transmitted by *Ixodes* ticks, via complicated, multi-stage growth (larvae / nymph / adult). **Each stage** must independently pick up the bacteria. Does **not** affect most hosts! Only humans / dogs / some cattle. ~476,000 people / year infected and treated in US. ] ??? Uninfected larvae hatch and seek a host to feed on, which is typically a small mammal or bird, but may include larger animals. Because *Borrelia burgdorferi* is not transmitted transovarially, this life stage is the primary opportunity for spirochaetes to infect ticks that feed on an infected host. After feeding, the six-legged larvae moult and emerge as eight-legged nymphs, which may be infected with spirochaetes acquired during their initial bloodmeal. Nymphs seek a second host, typically a small or medium-sized mammal, and this bloodmeal may offer a second opportunity for spirochaetes to infect ticks. Importantly, nymphs infected during the larval bloodmeal can transmit spirochaetes to hosts, including humans and domestic animals. After fed nymphs have moulted to the adult stage, newly emerged adult *Ixodes scapularis* ticks search for a large animal host, typically white-tailed deer, for mating and a final bloodmeal. Although deer are the preferred hosts, adult female ticks will also feed on humans and domestic animals, which can acquire *B. burgdorferi*, but are relatively unimportant to further perpetuation of infections. Because ticks cannot acquire *B. burgdorferi* from deer, these hosts are not effective reservoirs for *B. burgdorferi*, although they are important for perpetuation of tick populations. After mating, engorged females release themselves from hosts and eventually oviposit an egg mass, which may contain hundreds to thousands of eggs. I. scapularis ticks produce only a single clutch of eggs and then die. --- ## How to manage **Lyme Disease**? .pull-left-60[  > .large[It is called the **Deer tick** after all!] ] .pull-right-40[  ] --- .pull-left-60[ ## How to manage **Lyme Disease**?  #### But ... - Deer are dead-end hosts - Adult ticks on deer have do not shed bacteria - Fewer deer just means more nymphs per deer - Short of total elimination (impractical) very little effect ] .pull-right-30[ ### Experimental results  ] --- .pull-left[ ## Integrated tick management .small[ . | . --|-- Personal protection measures | Avoid tick habitats | Protective clothing | Tick checks and prompt tick removal | Synthetic chemical repellents | ‘Natural’ product-based repellents | Permethrin-treated clothing Treatment humans | Antibiotic use after tick bite | Human vaccine Landscape management | Xeroscaping/hardscaping | Remove leaf litter and brush, mow grass | Remove rodent harborage ([Stafford et al. 2017](https://academic.oup.com/jipm/article/8/1/28/4469309)) ]] .pull-right[.small[ . | . --|-- Target ticks |Synthetic chemical acaricides |Botanically-based acaricides | Biological agents and biopesticides (entomopathogenic fungi, nematodes, and other pathogens) | Acaricides with semiochemicals as lures or decoys Target rodents | Topical acaricide bait boxes | Oral Lyme disease vaccine bait | Oral antibiotic bait | Oral tick growth regulator Target deer | Topical acaricide self-treatment bait stations | Deer reduction | Systemic acaricides | Deer exclusion (fencing) | Oral tick growth regulator | Anti-tick vaccine ] .center[**Mainly: Manage People, not wildlife!**] ] --- ## **Lyme** and trophic interactions Fascinating hypothesis: The increase in Lyme is related to increase in coyotes!  In New York, observation rates from the bow-hunter wildlife survey indicate that Lyme disease incidence is **positively correlated with coyotes**, (B) **negatively correlated with foxes**, and (C) **unrelated to deer**. --- background-image: url("images/whitenose.jpg") background-size: cover # .center[**Wildlife diseases of Conservation Concern**] --- .pull-left-40[ ## White-nose Syndrome  ] .pull-right-60[ <br> **Pathogen**: *Pseudogymnoascus destructans* (fungus) > Not usually a good sign when your species name sounds like a malicious Transformer! Cold-loving fungus invades wing epidermis. Has caused collapse in many bat populations (local depletions ~ 90%). At least 12 North American species affected, mainly *Myotis spp.*.  ] --- .pull-left[ ## Rapid spread from NY epicenter ... to 38 states, 7 provinces, including appearance out west, since 2006.  **Note:** Endemic in bat populations in Europe and Asia, which have evolved resistance. ] .pull-right[ ## Most likely source Howe Caverns, New York State. Perhaps Spores on clothing from traveller from Europe?  ] --- ## How to manage **White-Nose Syndrome**? -- .pull-left-60[ - Intensive research - Intensive monitoring - Limit spread! - Outreach and communication - Search for vaccines / treatments  ] .pull-right-40[ - Cave closures - Caving protocols: - protective gear | heat treatment of outerwear | training  ] --- ## **Chytridiomycosis** .pull-left-60[  ] .pull-right-40[ **Pathogen**: *Batrachochytrium salamandrivorans* (chytrid fungus) Destroys skin, disrupts immune systems, causes heart failure. *Possibly* behaviorally modifies male frogs to be more attractive to female frogs. .center[] ] --- ## **Chytridiomycosis** *The chytridiomycosis panzootic represents the greatest recorded loss of biodiversity attributable to a disease.* [(Scheele et al. 2019)](https://www.science.org/doi/full/10.1126/science.aav0379). .pull-left-70[  - Main problem global transmission of disease - Possible contribution of **climate change** *increased cloud cover in drives convergence of daytime & nighttime temperatures towards thermal optimum* ([Rohr et al. 2008](https://www.pnas.org/doi/10.1073/pnas.0806368105)) ] .pull-right-30[  Keywords: **Global Pangaea** | **Biosecurity** | **Intensive Monitoring** ] --- class: middle ## COVID-19 in wildlife .center[**pseudo guest lecture by Dr. Tricia Fry**] .pull-left-30[<br><br><br><br><br>] .pull-right-70[] --- ## The main tool: **Wildlife Surveillance** .large.blue[Systematic on-going collection, collation and analysis of information related to animal health and timely dissemination of information to those who need to know so action can be taken. (*World Organization of Animal Health (WOAH)*) ] - **Continuous:** Vigilance and investigation - Regular analysis of data (to detect trend) For example: avian influenza detected: 1. investigating mortality events, 2. using hunter-harvested samples, 3. live bird sampling - Constant Communication of results - publication + dissemination (via agencies) > Excellent video: **https://www.youtube.com/watch?v=vSNsi4Cj0k4&ab_channel=CWHLCornell** --- class: inverse ## Other **Tools**: .pull-left-30[ .large[ 1. Surveillance 2. Prevention 3. Research 3. Training 4. Teaching 5. Policy Support ] ] .pull-right-70[] --- background-image: url("images/LEGO_bg.jpg") background-size: cover class: middle # .white.large.center[**Disease modeling**] --- ## Disease modeling ### Everyone in a population of fixed size `\(N\)` is either: .pull-left[ - **Susceptible**, who (can become) - **Infected** after which they (can become) - **Removed**. (**Removed** are either **Recovered** or **Recently Deceased**) ] `$$\left\{\begin{aligned} & \frac{dS}{dt} = - \frac{\beta I S}{N}, \\[6pt] & \frac{dI}{dt} = \frac{\beta I S}{N}- \gamma I, \\[6pt] & \frac{dR}{dt} = \gamma I, \end{aligned}\right.$$` Note similarity (simplification) relative to **predator-prey** models. Sum of the three rates `\({dS \over dt} + {dI \over dt} + {dR\over df} = 0\)`, because population is fixed at `\(S+I+R = N\)` (none of the crazy fluctuations of Lotka-Volterra). --- ## SIR Model Graphical  Eventually EVERYONE will be Removed! (good news? bad news?) --- ## Compartmental Models Getting more Complex .pull-left[  [Getz et al. 2017](https://www.biorxiv.org/content/10.1101/191601v2.abstract) ] .pull-right[ <img src='images/COVID_models.png' width='100%'/> [Khan et al. 2020](https://doi.org/10.1017/S0950268820002423) ] SQUIRED model --- class: inverse ## Pseudo-distinguished Guest Lecture on Disease Modeling .center[] <!-- ## Most important diseases for NY professionals .pull-left[ **Vector-borne** - Rabies - Brucellosis - Tuberculosis - Avian Influenza - Tularemia - West Nile visyt (mosquito) - Lyme disease (black-legged tick) - Eastern equine encephalitis (mosquito) ] .pull-right[ - canine distemper - mange ] -->